testing water acidity between tap and bottled study|alkaline water ph test : supermarket This study offers a comparative analysis of bottled water, household-treated tap water, and tap water quality using a unique dataset to evaluate potential organoleptic and toxicity trade-offs among drinking water options for households in the San Francisco Bay Area. Resultado da Topo de bolo ano novo 2024 pronto para imprimir. Topo de bolo ano novo 2024, preparamos alguns modelos de topos para você usar nessa virada de ano, e começa o novo ano da melhor forma possível. Nosso topo de bolo feliz ano novo é totalmente gratuito, é só você os baixar aqui no blog. .
{plog:ftitle_list}
Resultado da 1 de ago. de 2019 · O Globoplay, serviço de streaming por assinatura da Globo, divulgou a série Todo Mundo Odeia o Chris entre as estreias de agosto na plataforma em suas redes sociais nesta quinta-feira, 1º. A .
This study offers a comparative analysis of bottled water, household-treated tap water, and tap water quality using a unique dataset to evaluate potential organoleptic and toxicity trade-offs among drinking water options for households in the San Francisco Bay Area.The recommended pH range for drinking water is typically between 6.5 and 8.5. Alkalinity: Pure water has a limited ability to buffer against changes in Ph. Alkalinity is crucial for maintaining the stability of water pH. It acts as a buffer, . The Environmental Protection Agency (EPA) recommend keeping the pH between 6.5 and 8.5 in drinking water, and many states in the United States choose to enforce these levels. Risks and benefits of .Temperature will also affect the equilibria and the pH. In pure water, a decrease in pH of about 0.45 occurs as the temperature is raised by 25 °C. In water with a buffering capacity imparted by bicarbonate, carbonate and hydroxyl ions, this temperature effect is modified (APHA, 1989). The pH of most drinking-water lies within the range 6.5–8.5.
Compared with the consumption of other unimproved water sources and tap water, drinking bottled water could lower the risk of diarrhoea (Komarulzaman et al. 2019) and water lead exposure (Pieper et al. 2019). . Almost 75% of the bottled waters tested in the present study were so acidic that they could dissolve enamel. Furthermore, the pH .
Most DIY water testing kits contain test strips and a color wheel or chart. Here’s how to test your water using a DIY water test kit: Take a drinking water sample from your faucet in a clean cup or container; Unbox your home test kit and dip a test strip into the water; Wait for the squares on the test strip to change colorThe pH of bottled water is a significant concern. The bottled water industry often uses ozone, a highly acidic substance, for pathogen removal. As a result, the pH of ozone-treated bottled water is more acidic than its source water counterpart, highlighting the importance of maintaining a balanced pH in our drinking water.
This study is to evaluate water quality of tap water, storage water, filtered water, and filtered water dispenser. The water samples from 2,354 attending places are collected and analyzed. The pH level also affects the availability of essential plant nutrients, with many nutrients being less available at a pH above 7 . pH and Drinking Water. There is no legally enforceable standard for drinking water pH levels because pH is considered an aesthetic water quality. However, the U.S. Environmental Protection Agency (EPA) recommends a .
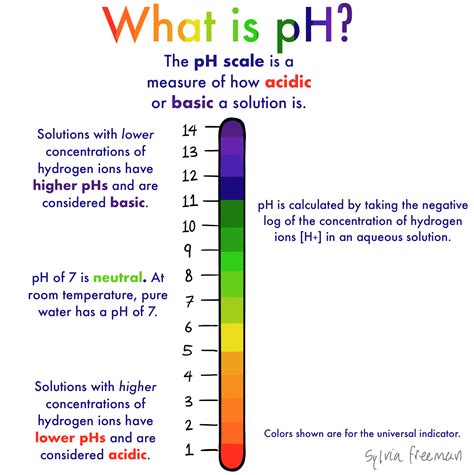
ph test for water
At least 45% of the nation’s tap water is estimated to have one or more types of the chemicals known as per- and polyfluorinated alkyl substances, or PFAS, according to a new study by the U.S. Geological Survey. There are more than 12,000 types of PFAS, not all of which can be detected with current tests; the USGS study tested for the presence of 32 types. Advances in drinking water infrastructure and treatment throughout the 20th and early 21st century dramatically improved water reliability and quality in the United States (US) and other parts of . Understanding drinking water quality at the point-of-use across a range of consumer options is essential for designing effective public health interventions in the face of deteriorating source waters and complex contaminant mixtures. This is especially pressing as the popularity of tap water alternatives like bottled water and household treatment increases, yet .
Tap and bottled water can be tested with pH, chlorine and nitrates/nitrites test strips, which come with their own color chart for comparison. Pour equal amounts (two to three ounces) of bottled water and tap water in clear containers. First, test each sample with 4.5 to 7.0 pH strips for acidity and then with 6.5 to 10 pH strips for alkalinity.
Journal of Comparative Study on the Drinking Water Quality of Tap Water, Bottled Water and Water From . water dispenser,tap water,bacteriological test, pH and . sanitation practices of the .

Brands of bottled water tend to aim for the ideal pH range for drinking water, which is typically between 6.5 and 8.5, since this gives water the most balanced taste. For the sake of comparison, tap water in the US has a pH range of 6.5 and 9.5, depending on . The alkalinity of water refers to its pH, which is measured on a scale of zero to 14 based on its relative alkaline (basic) or acidic nature. A liquid with a pH of seven is considered neutral.A clinical trial by Koufman and Johnston found naturally occurring alkaline water (pH 8.8) to be therapeutic in the treatment of acid reflux disease. 13 Alkaline water was found to denature human pepsin 3b, which is the enzyme that .A pH less than 7 is acidic; a pH greater than 7 is alkaline. The accompanying data represent the pH in samples of bottled water and tap water. Find the Mean, Median and Mode for each type of water in table A only. . Based on a recent study, the pH level of the arterial cord (one vessel in the umbilical cord) is normally distributed with mean .
The ideal pH for drinking water is between 6.5 and 8.5, with 7 being considered neutral. . Using a pH meter is a simple and effective way to test the pH of your drinking water. The pH meter used in the study is a . The permissible range of pH of Drinking water between 6.5 and 8.5 according to WHO. If the pH is below 6.5it is considered to be Acidic water tends to be corrosive to pipes and hand pumps. pH above 8.5 is Alkaline water and may tend to have a bitter or soda-like taste (Goon et al., 1986). Bicarbonate levels in tap water varied by a factor of 5.12, where levels were highest in Perth with 61.4 mg/L and lowest in Cairns with 12 mg/L. Bottled water tended to be a more abundant source, and even comparing to the most bicarbonate rich tap water, bottled still and sparkling water had a 2.1‐ and 3.8‐fold higher bicarbonate content .The pH of the water is an indicator of the hydrogen ion concentration present in it. A pH of 0 means the solution is a strong acid; a pH of 14 means it is a strong base. . Tap water; Bottled water; Water test kit (The kit should test for pH, nitrate and nitrite concentrations, total hardness, free chlorine, total chlorine, iron concentrations .
Depend on WHO standard in 2006 all the samples were considered to be soft (from 40 to 65 mg/l) except for the University area which Table I Illustrates pH for the Samples of Both Tap and Bottled Water Samples November December January February March April Bottled water B1 B2 B3 B4 Tap water General Hospital Iskan Hamamok Koya University 6.2 6.5 . The EPA estimates that between 6 and 10 percent of the 66,000 public drinking water systems in the U.S. will find PFAS once they start testing. If you’re on well water, you won’t get a CCR, so . Study design and randomization. A randomized, double-blind water taste test was designed (Fig. 1).Water from a public drinking fountain (tap water) and cold water from a POU water dispenser . The results revealed that pH fluctuations had a substantial influence on these parameters, indicating a direct relationship between pH levels and water quality. The study findings contribute to .
There are many reasons why companies and scientists test pH levels in water. For drinking water, the pH range must be between 6.5-8.5. If the water is too acidic, it will likely taste and smell bad. It can also cause illness depending on the types of contaminants that are found in the water.
To test their new high-throughput imaging platform, the team analyzed the micro- and nanoplastics in three popular brands of bottled water. Results were reported on January 8, 2024, in the Proceedings of the National Academy of Sciences. The researchers found that, on average, a liter of bottled water included about 240,000 tiny pieces of plastic.
In this article, the authors explore the demographic and social factors associated with bottled water users in the U.S. and the relationship between bottled water use and perceptions of the quality of local water supply. A brief discussion of bottled water and tap water and bottled water consumers is used to develop several hypotheses.
half shade polarimeter pdf
half shade polarimeter principle
Resultado da Em 'O Menino e a Garça', a jornada de Mahito se transforma em um conto mágico após a perda de sua mãe. Ao explorar a propriedade da família, ele se depara com uma torre antiga, desencadeando eventos misteriosos. Quando sua madrasta desaparece, Mahito segue a travessa garça .
testing water acidity between tap and bottled study|alkaline water ph test